KDEL sign peptide together with focusing on affibody molecules improves cytotoxicity of RTA towards goal most cancers cells
Human epidermal development issue receptor 2 (HER2) and epidermal development issue receptor (EGFR; HER1) are consultant most cancers markers which can be overexpressed on the floor of a number of most cancers cells [24, 25]. Subsequently, we used affibodies that concentrate on HER2- or EGFR-overexpressing most cancers cells [21] whereas designing our RITs. The coding sequence of an affibody molecule that binds to HER2 (ZHER2:342; HER2Afb) or EGFR (ZEGFR:1907; EGFRAfb) [26, 27] was genetically fused to the N-terminus of RTA (residues 36–302) to acquire RTA-based RIT clones (Extra file 1: Fig. S2A and B). The constructed HER2Afb-RTA and EGFRAfb-RTA clones have been overexpressed in E. coli BL21 (DE3) pressure and purified by immobilized metallic affinity chromatography (Extra file 1: Fig. S2C).
Subsequent, we carried out cell viability assays to quantitatively look at target-specific cytotoxicity of every RIT. We selected NIH3T6.7 (immortalized mouse fibroblast cell) and SK-BR-3 (human breast most cancers cell) as HER2-overexpressing most cancers cell traces, and A431 (human pores and skin most cancers cell) and MDA-MB-468 (human breast most cancers cell) as EGFR-overexpressing most cancers cell traces. We handled them with HER2Afb-RTA or EGFRAfb-RTA. Nonetheless, cytotoxic results of the RITs towards their corresponding goal cells have been a lot decrease than anticipated (Extra file 1: Fig. S1). Whereas the HER2Afb-RTA confirmed greater cytotoxicity towards NIH3T6.7 cells than doxorubicin did, EGFRAfb-RTA exhibited related cytotoxicity as doxorubicin towards MDA-MB-468 cells (Extra file 1: Fig. S1A and C). As well as, each the RITs confirmed nearly no cytotoxicity towards SK-BR-3 and A431 cells (Extra file 1: Fig. S1B and D). The RTB is thought to not solely bind to its receptor but in addition retrogradely transport the RTA to the ER [8] and substitute of the RTB with both HER2Afb or EGFRAfb might alter the vacation spot of the RTA, ensuing within the vital lack of cytotoxicity.
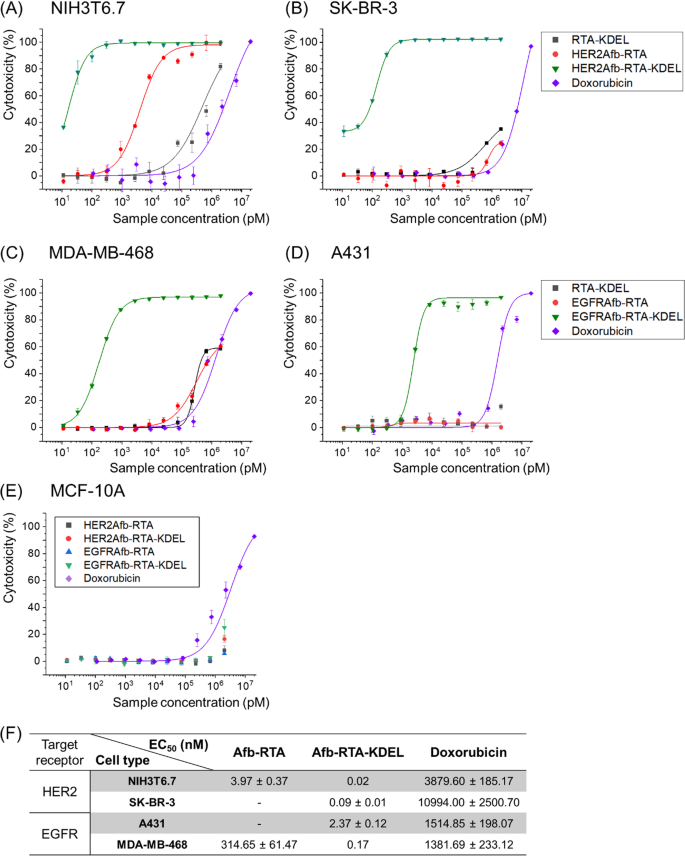
To revive the ER-targeting-capability, we genetically launched the KDEL sign peptide [22, 23] on the C-termini of HER2Afb-RTA and EGFRAfb-RTA (Extra file 1: Fig. S2). We measured cell cytotoxicity in a dose-dependent method with a cell counting kit-8 assay (Fig. 1A–E) and calculated EC50 values of every immunotoxin towards its corresponding goal cell [28] (Fig. 1F). As we hypothesized, addition of the KDEL sign peptide considerably improved the cytotoxicity of each the RITs (Fig. 1, inexperienced triangles vs. purple circles). The EC50 worth of HER2Afb-RTA-KDEL towards NIH3T6.7 cells diminished from 3.97 to 0.02 nM, and that of EGFRAfb-RTA-KDEL towards MDA-MB-468 cells diminished from 314.65 to 0.17 nM (Fig. 1). Extra surprisingly, each the KDEL-containing RITs confirmed dramatically enhanced cytotoxicity towards SK-BR-3 and A431 cells which have been proof against the non-KDEL-containing RITs (Fig. 1, inexperienced triangles). Nonetheless, MCF-10A, which is a non-tumorigenic regular epithelial cell line that neither overexpresses HER2 nor EGFR, didn’t exhibit any noticeable cell demise upon therapy with the RITs, implying that their mode of motion was cancer-specific (Fig. 1E). Cells have been additionally handled with RTA-KDEL, with out the focusing on ligands, to analyze the impact of KDEL alone (Fig. 1A–D; black squares). RTA-KDEL confirmed average cytotoxicity towards NIH3T6.7 cells, decrease than that of HER2Afb-RTA and HER2Afb-RTA-KDEL. Within the case of MDA-MB-468 cells, RTA-KDEL confirmed related cytotoxicity to that of EGFRAfb-RTA (Fig. 1A and C; black squares and purple circles). In distinction, RTA-KDEL didn’t present any noticeable cytotoxicity towards SK-BR-3 and A431 cells, like HER2Afb-RTA and EGFRAfb-RTA (Fig. 1B and D; black squares and purple circles). These outcomes recommend that the cytotoxicity of RTA-KDEL is correlated with the goal cell’s sensitivity towards HER2Afb-RTA or EGFRAfb-RTA. Though many cells have KDEL receptors on their floor, and RTA-KDEL might bind to them and direct the RTA to ER, SK-BR-3 and A431 cells have been nonetheless proof against RTA-KDEL implying that binding to KDEL receptors of SK-BR-3 and A431 cells wouldn’t be ample to kill them. Ricin-based RITs constructed right here confirmed variable cytotoxicity towards totally different cell traces, however their cytotoxic efficacy was maximized when each the focusing on ligand and the KDEL sign peptide have been current.
In vitro measurements of dose-dependent cytotoxicity. Dose-dependent cytotoxicity of RTA-KDEL, HER2Afb-RTA, HER2Afb-RTA-KDEL and doxorubicin in HER2-overexpressing A NIH3T6.7 and B SK-BR-3 cells; RTA-KDEL, EGFRAfb-RTA, EGFRAfb-RTA-KDEL and doxorubicin in EGFR-overexpressing C MDA-MB-468 and D A431 cells; HER2Afb-RTA, HER2Afb-RTA-KDEL, EGFRAfb-RTA, and EGFRAfb-RTA-KDEL in E MCF-10A, utilizing CCK-8 assay. F Half-maximal efficient focus (EC50) values of HER2Afb-RTA, HER2Afb-RTA-KDEL, EGFRAfb-RTA, EGFRAfb-RTA-KDEL, and doxorubicin towards every cell. Curves have been fitted utilizing a Hill equation to find out EC50 values. All knowledge proven are means ± normal deviation (SD); n = 3
HER2Afb-RTA-KDEL and EGFRAfb-RTA-KDEL induce efficient apoptosis of their corresponding goal most cancers cells
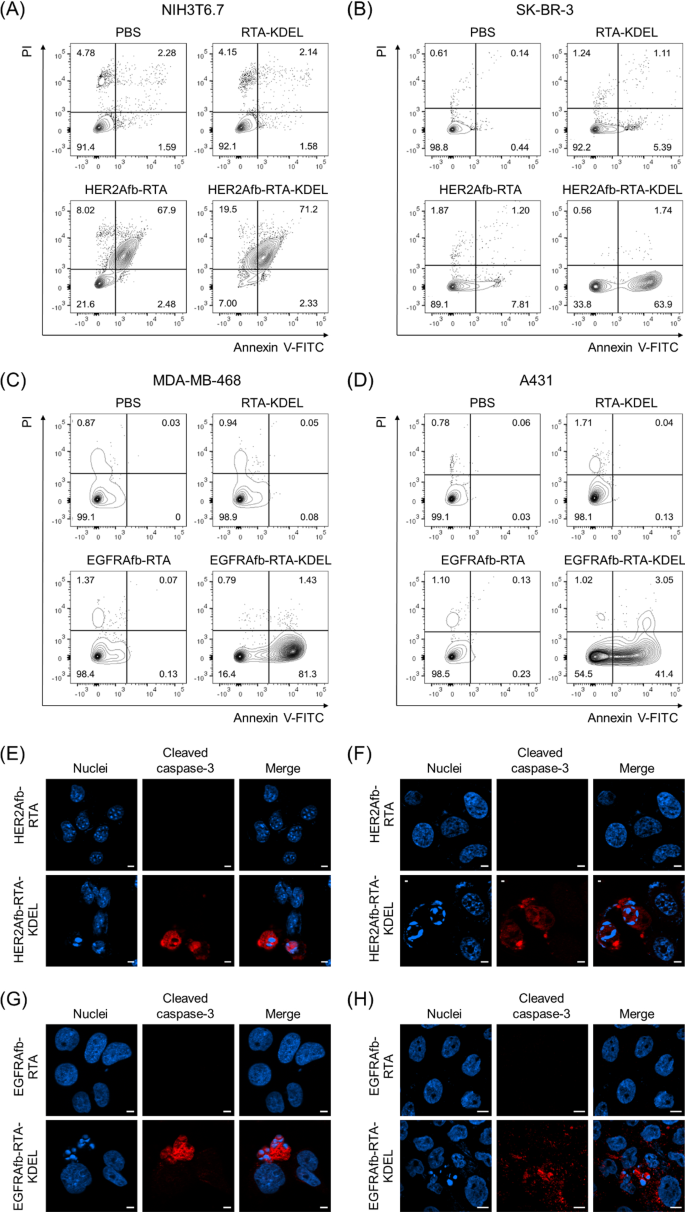
Ricin holotoxin induces apoptosis by inactivating the ribosome and arresting protein synthesis in cells [10, 11]. We investigated whether or not the KDEL-containing RITs may induce target-specific apoptosis utilizing Annexin V/propidium iodide (PI) staining, which measures apoptosis quantitatively based mostly on the diploma of cell membrane integrity. NIH3T6.7 and SK-BR-3 cells have been challenged with 0.5 μM of RTA-KDEL, HER2Afb-RTA, or HER2Afb-RTA-KDEL; MDA-MB-468 and A431 cells have been challenged with the identical quantities of RTA-KDEL, EGFRAfb-RTA, or EGFRAfb-RTA-KDEL. Cells have been then stained with annexin V-FITC and PI 48 h after pattern therapy to forestall full cell demise in all populations. In all cell traces handled with phosphate-buffered saline (PBS), annexin V/PI double unfavorable cells have been dominant (Fig. 2A–D, decrease left quadrants) indicating that the cells have been alive. Both HER2Afb-RTA or HER2Afb-RTA-KDEL-treated NIH3T6.7 cells confirmed roughly 70% of annexin V/PI double optimistic populations, implying that the cells had undergone late apoptosis already (Fig. 2A, higher proper quadrant). In step with the cell viability knowledge, each HER2Afb-RTA and HER2Afb-RTA-KDEL successfully induced apoptosis of NIH3T6.7 cells whatever the KDEL sign peptide. Whereas 63.9% of SK-BR-3 cells handled with HER2Afb-RTA-KDEL have been annexin V optimistic, however PI unfavorable (decrease proper quadrants), indicating that they have been in early apoptosis, solely 7.81% shifted to an early apoptotic state upon HER2Afb-RTA therapy (Fig. 2B). Equally, 81.3% of MDA-MB-468 cells handled with EGFRAfb-RTA-KDEL have been in early apoptotic state, whereas 98.4% of cells have been alive upon EGFRAfb-RTA or RTA-KDEL therapies (Fig. 2C). A431 cells additionally started to shift to early apoptotic section solely after the therapy of EGFRAfb-RTA-KDEL and 41.4% of cells have been annexin V optimistic, however PI unfavorable at 48 h incubation (Fig. 2D). MDA-MB-468 cells would possibly shift to the apoptotic state extra rapidly than A431 cells, as a result of MDA-MB-468 cells have been extra inclined to EGFRAfb-RTA-KDEL than A431 cells (Fig. 1C, D, F; EC50 ~ 0.17 vs. 2.37 nM) and the move cytometry analyses have been carried out at shorter timepoint (48 h) and decrease pattern focus (0.5 μM) than the cell viability check to forestall full cell demise (Fig. 2).
Circulation cytometry and immunocytochemical evaluation of cells handled with totally different RITs. A NIH3T6.7 and B SK-BR-3 cells have been handled with PBS, RTA-KDEL, HER2Afb-RTA, or HER2Afb-KDEL. C MDA-MB-468 and D A431 cells have been handled with PBS, RTA-KDEL, EGFRAfb-RTA, or EGFRAfb-RTA-KDEL. In every plot, the higher left and proper quadrants signify necrosis and late apoptosis, respectively, and the decrease left and proper quadrants signify regular state of cells and early apoptosis, respectively. Relative populations of every quadrant are individually marked. Immunocytochemical detection of cleaved caspase-3 (purple) and nuclei (blue) in: E NIH3T6.7 and F SK-BR-3 cells handled with HER2Afb-RTA or HER2Afb-RTA-KDEL; G MDA-MB-468 and H A431 cells handled with EGFRAfb-RTA or EGFRAfb-RTA-KDEL. Scale bars, 10 μm
To additional verify target-specific HER2Afb- or EGFRAfb-RTA-KDEL-induced apoptotic cell demise at a lot decrease focus, cleaved caspase-3, a well-studied apoptosis marker, was visualized within the cells. Every cell line was handled with 5 nM of corresponding HER2Afb-RTA, EGFRAfb-RTA, HER2Afb-RTA-KDEL, or EGFRAfb-RTA-KDEL, after which stained with anti-cleaved caspase-3 antibody conjugated with a fluorescent dye (Fig. 2E–H). NIH3T6.7 and SK-BR-3 cells handled with HER2Afb-RTA-KDEL, and MDA-MB-468 and A431 cells handled with EGFRAfb-RTA-KDEL confirmed the fluorescent indicators of cleaved caspase-3 (Fig. 2E–H, purple) with fragmented nuclei, whereas there was no fluorescent sign or fragmented nuclei in different cells handled with both HER2Afb-RTA or EGFRAfb-RTA. These outcomes recommend that RITs with the KDEL sign peptide (HER2Afb-RTA-KDEL and EGFRAfb-RTA-KDEL) efficiently induced apoptosis in a target-dependent method, resulting in efficient most cancers cell demise.
KDEL sign peptide permits efficient intracellular supply of the RTA
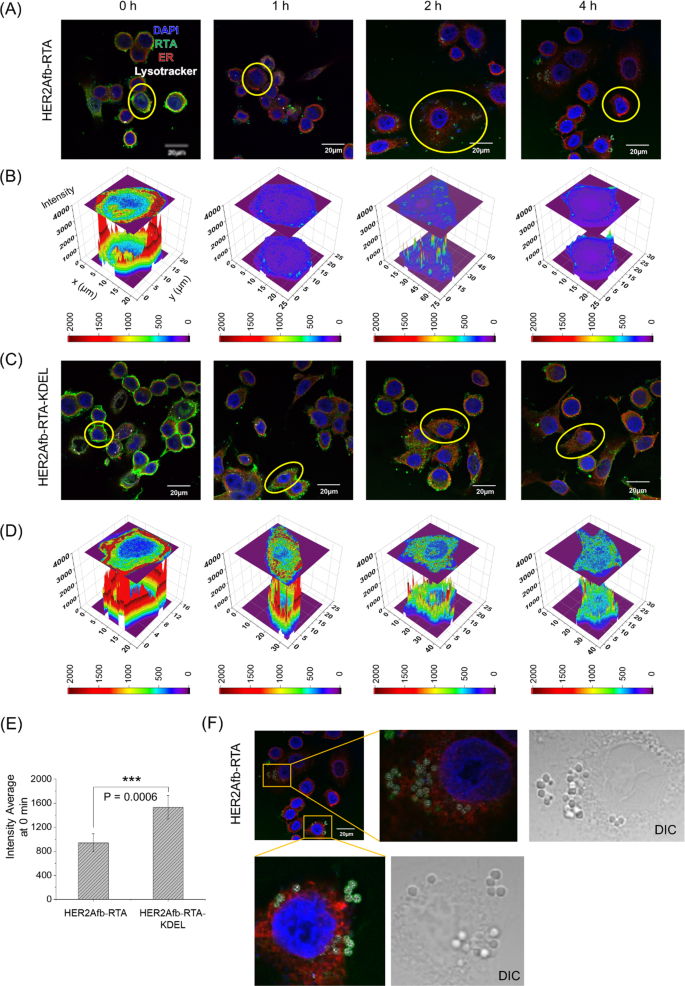
To discover how the KDEL sign peptide enhances cytotoxicity, we first handled SK-BR-3 cells with both HER2Afb-RTA or HER2Afb-RTA-KDEL and visualized the intracellular localization of the RTA in every cell after varied incubation occasions by fluorescence microscopy (Fig. 3, inexperienced in photos). Each HER2Afb-RTA and HER2Afb-RTA-KDEL selectively certain to the floor of SK-BR-3 cells proper after the therapy (Fig. 3A and C, 0 h). Nonetheless, HER2Afb-RTA-KDEL certain extra intensely than HER2Afb-RTA (Fig. 3E), in all probability attributable to surface-presented KDEL receptors on SK-BR-3 cells [29, 30]. HER2Afb-RTA dramatically disappeared from the cells with time, and was discovered outdoors the cells (Fig. 3A), whereas HER2Afb-RTA-KDEL was visualized contained in the cells even after 4 h (Fig. 3C). Quantitative fluorescence analyses of the RTA revealed that a lot of the HER2Afb-RTA was faraway from the cells inside 1 h (Fig. 3B), whereas a lot of the HER2Afb-RTA-KDEL remained within the cytosol even after 4 h (Fig. 3D). Curiously, we may observe budding microvesicle-like constructions, which have the RTA and lysotracker sign collectively, solely in SK-BR-3 cells handled with HER2Afb-RTA (Fig. 3A and F).
Fluorescence photos of SK-BR-3 cells handled with A HER2Afb-RTA or C HER2Afb-RTA-KDEL at varied time factors. Nuclei, RTA, ER, and lysosomes are proven in blue, inexperienced, purple, and white, respectively. Scale bars, 20 μm. One of many cells in A and C was chosen and fluorescence depth profiles of B HER2Afb-RTA and D HER2Afb-RTA-KDEL within the chosen space have been analyzed. The fluorescence depth of RTA is represented by a colorimetric scale bar, and 2D projection of the world was drawn on high of the 3D plot. E Fluorescence sign depth of HER2Afb-RTA or HER2Afb-RTA-KDEL certain to the cell floor at 0 h was statistically measured by line depth evaluation and plotted as a bar graph. All knowledge are proven as means ± SD; ***p < 0.001. F Microvesicle-like constructions containing RTA on the floor of SK-BR-3 cells handled with HER2Afb-RTA have been enlarged with DIC photos
NIH3T6.7 cells have been additionally handled with both HER2Afb-RTA or HER2Afb-RTA-KDEL and intracellular localization of the RTA was monitored. Much like the observations in SK-BR-3 cells, HER2Afb-RTA-KDEL certain extra strongly to NIH3T6.7 cell floor and remained longer within the cytosol than HER2Afb-RTA (Extra file 1: Fig. S3). Nonetheless, in contrast to in SK-BR-3 cells, vital quantities of HER2Afb-RTA have been nonetheless noticed within the cytosol all through the experiment and no microvesicle-like constructions have been noticed within the cells (Extra file 1: Fig. S3A). These outcomes suggest that HER2Afb-RTAs selectively bind to HER2-overexpressing SK-BR-3, however they could be recycled again to the cell floor to be eliminated via microvesicle-like constructions, making SK-BR-3 cells proof against HER2Afb-RTA. Nonetheless, NIH3T6.7 cells weren’t proof against HER2Afb-RTA, in all probability as a result of they lack a recycling equipment within the HER2-mediated endocytosis pathway, permitting HER2Afb-RTA to successfully enter the cells and induce apoptosis. The cytotoxicity in NIH3T6.7 cells might primarily rely on the preliminary quantities of the certain RITs quite than the presence of the KDEL sign peptide.
MDA-MB-468 cells have been additionally handled with EGFRAfb-RTA or EGFRAfb-RTA-KDEL, to analyze focusing on ligand-dependency of RITs with or with out the KDEL sign peptide. EGFRAfb-RTA-KDEL additionally certain to the cell floor extra and remained longer within the cytosol (Extra file 1: Fig. S4A–E), justifying our rationale that the addition of the KDEL sign peptide to immunotoxins would improve their goal cell-binding capability and intracellular supply effectivity, whatever the focusing on ligand species. Small quantities of EGFRAfb-RTA nonetheless remained inside MDA-MB-468 cells till 2 h post-treatment and disappeared fully by 4 h (Extra file 1: Fig. S4A and B), supporting the average cytotoxicity of EGFRAfb-RTA towards these cells (Fig. 1C). We may additionally observe surface-budding microvesicles of RTA in MDA-MB-468 cells, like these in HER2Afb-RTA-treated SK-BR-3 cells (Extra file 1: Fig. S4F). These outcomes suggest that MDA-MB-468 cells additionally recycle EGFRAfb-RTA however much less effectively than SK-BR-3 cells, probably ensuing of their average sensitivity to EGFRAfb-RTA. These knowledge strongly recommend that the KDEL sign peptide improves the cytotoxicity of each the RITs by circumventing a recycling pathway in addition to directing them to the retrograde transport pathway to succeed in the ER to be delivered to the cytosol.
KDEL sign peptide permits the RTA to avoid endosomal recycling
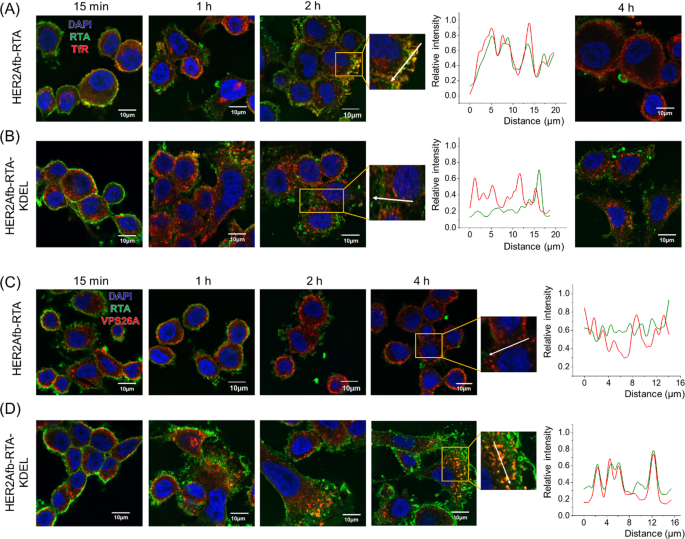
To additional examine which pathway HER2Afb-RTA or HER2Afb-RTA-KDEL adopted when HER2Afb-RTA-resistant cells, resembling SK-BR-3 cells, have been handled with them, we visualized the RTA in cells with a fluorescence microscope and in contrast their places with transferrin receptors (TfR) [31] and VPS26A [32], that are recycling endosome and retromer markers, respectively. The fluorescent indicators of HER2Afb-RTA (inexperienced) overlapped with these of TfR (purple) 2 h after pattern therapy, however fully disappeared 4 h after therapy (Fig. 4A); the fluorescent indicators of HER2Afb-RTA-KDEL (inexperienced) and TfR (purple) didn’t overlap, and each remained contained in the cells even 4 h after therapy (Fig. 4B). These knowledge recommend that the elimination of HER2Afb-RTA noticed in Fig. 3A is as a result of recycling pathway performing between 2 and 4 h after pattern therapy.
Fluorescence photos of SK-BR-3 cells handled with HER2Afb-RTA (A, C) or HER2Afb-RTA-KDEL (B, D) at varied time factors. A, B Nuclei, RTA, and TfR are proven in blue, inexperienced, and purple, respectively. C, D Nuclei, RTA, and VPS26A are proven in blue, inexperienced, and purple, respectively. Co-localization of RTA and TfR (A, B at 2 h) or RTA and VPS26A (C, D at 4 h) was additionally analyzed with a line depth profile in keeping with distance. The analyzed traces are indicated as white arrows. Scale bars, 10 μm
In distinction, we may observe the fluorescent indicators of HER2Afb-RTA-KDEL (inexperienced) co-localizing with these of VPS26A (purple) from 2 h post-treatment and lasting until 4 h after therapy (Fig. 4D). Nonetheless, the fluorescent indicators of HER2Afb-RTA (inexperienced) didn’t overlap with these of VPS26A, and so they largely disappeared after 4 h post-treatment (Fig. 4C). These outcomes recommend that the addition of the KDEL sign peptide can overcome resistance of SK-BR-3 cells to HER2Afb-RTA, by switching from a recycling to a retrograde transport pathway, which appears to be a slower course of (Fig. 4).
EGFRAfb-RTA and EGFRAfb-RTA-KDEL have been additionally trafficked with TfR and VPS26A in MDA-MB-468 cells. Overlapping fluorescent indicators of EGFRAfb-RTA (inexperienced) and TfR (purple) have been noticed 2 h after pattern therapy (Extra file 1: Fig. S5A). The fluorescent indicators of EGFRAfb-RTA-KDEL (inexperienced) didn’t co-localize with these of TfR (purple), however they overlapped with these of VPS26A (purple) between 2 and 4 h after pattern therapy. Nonetheless, the fluorescent indicators of EGFRAfb-RTA didn’t co-localize with these of VPS26A, once more supporting the idea that the KDEL sign peptide re-routes the RTA to the retrograde pathway (Extra file 1: Fig. S5B).
Albumin-binding area and protein ligation system have been launched for in vivo anti-tumor experiment
Since HER2Afb-RTA-KDEL and EGFRAfb-RTA-KDEL confirmed glorious target-specific intracellular RTA supply with low EC50 values in vitro, we determined to discover their in vivo efficacy as nicely. Nonetheless, the small-sized RITs could be rapidly cleared from circulation upon the systemic administration, earlier than reaching the tumor web site [33]. To extend blood circulation time and improve in vivo therapeutic efficacy, we genetically launched the beforehand developed albumin-binding area (AlBD) [18, 19, 34,35,36] to the N-terminus of RTA-KDEL, establishing AlBD-RTA-KDEL. We and different teams beforehand confirmed that the AlBD-fused proteins bind with excessive affinity to human and mouse serum albumins in blood [15, 33], and keep roughly 10 occasions longer in blood than these with out AlBD [33].
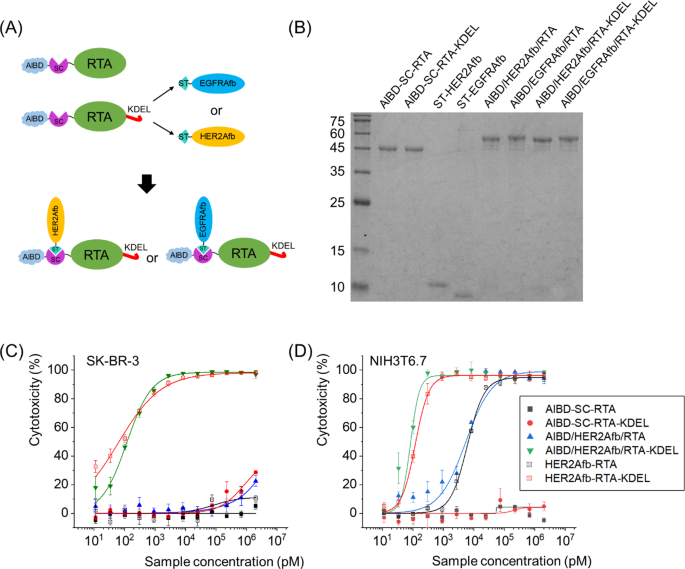
As well as, we genetically inserted SpyCatcher protein between AlBD and the RTA as illustrated in Fig. 5A. The SpyCatcher (SC) and SpyTag (ST) are cut up types of a fibronectin-binding protein derived from Streptococcus pyogenes and so they kind a spontaneous and irreversible isopeptide bond upon recognizing one another [37]. We will make the most of this post-translational ligation between SC and ST to modularly assemble SC-fused RTA and ST-fused affibody molecules collectively. The utilization of SC/ST would permit us to (i) bypass steric hindrance that AlBD exerts on the perform of focusing on ligand, (ii) give the toxin target-switchability, and (iii) alter configuration of immunotoxins as Y-shape which makes them bulkier [18].
Building of AlBD-SC-RTA-KDEL by genetic fusion of AlBD and SC, and subsequent goal ligand addition through an SC/ST protein ligation system. A Schematic illustration and B SDS-PAGE evaluation of post-translationally ligated resultants between AlBD-SC-RTA (50.0 kDa) and ST-HER2Afb (10.3 kDa) or ST-EGFRAfb (10.1 kDa), or between AlBD-SC-RTA-KDEL (50.4 kDa) and ST-HER2Afb or ST-EGFRAfb, forming AlBD/HER2Afb/RTA (60.3 kDa), AlBD/EGFRAfb/RTA (60.1 kDa), AlBD/HER2Afb/RTA-KDEL (60.7 kDa) and AlBD/EGFRAfb/RTA-KDEL (60.5 kDa), respectively. In vitro measurements of dose-dependent cytotoxicity of AlBD-containing RITs in C SK-BR-3 and D NIH3T6.7 cells, utilizing CCK-8 assay. Every assemble was indicated
Newly constructed AlBD-SC-RTA-KDEL was efficiently ligated with ST-fused HER2Afb or EGFRAfb to kind AlBD/HER2Afb/RTA-KDEL or AlBD/EGFRAfb/RTA-KDEL (Fig. 5A and B). They exhibited nearly an identical target-specific cytotoxicity in vitro to the values measured for HER2Afb-RTA-KDEL or EGFRAfb-RTA-KDEL (Fig. 5C, D and Extra file 1: Fig. S6). AlBD-SC-RTA with out the KDEL sign peptide was additionally constructed, ligated with both ST-fused HER2Afb or EGFRAfb, and examined as controls (Fig. 5B–D and Extra file 1: Fig. S6).
AlBD/HER2Afb/RTA-KDEL considerably suppresses development of tumor proof against HER2Afb-RTA solely
We established two in vivo mouse fashions, NIH3T6.7- and SK-BR-3-tumor-bearing mice, as a result of they confirmed totally different responses to immunotoxins with the identical focusing on ligands with or with out the KDEL sign peptide. We first investigated the anti-tumor results of RTA variants on NIH3T6.7 tumor-bearing mice. NIH3T6.7 cells have been allografted to immunodeficient nude mice and 5 μg of AlBD/HER2Afb/RTA-KDEL, AlBD/HER2Afb/RTA, AlBD-SC-RTA-KDEL or PBS have been intravenously injected when the typical tumor quantity reached 100 mm3, roughly. Every group was injected each two or three days for eight occasions and physique weights and tumor volumes have been measured earlier than injection (Extra file 1: Fig. S7A and B). Mice have been sacrificed at day 28, and main organs (coronary heart, lung, liver, kidney, and spleen) and tumor plenty have been biopsied (Extra file 1: Fig. S7C–E). In step with the in vitro knowledge, AlBD/HER2Afb/RTA-KDEL and AlBD/HER2Afb/RTA noticeably suppressed the expansion of NIH3T6.7 tumors, however AlBD/HER2Afb/RTA-KDEL exhibited a superior suppression impact with none vital side-effects to regular tissue (Extra file 1: Fig. S7). Nonetheless, AlBD-SC-RTA-KDEL didn’t have an effect on the expansion of the tumors in contrast with controls (Extra file 1: Fig. S7A–D), suggesting that the focusing on ligand is important for in vivo efficacy. Though the in vivo tumor suppression efficacy of HER2Afb-RTA-KDEL and HER2Afb-RTA was statistically vital, the in vivo cytotoxic efficacy of each AlBD/HER2Afb/RTA-KDEL and AlBD/HER2Afb/RTA was not adequate to fully suppress tumor development in all probability as a result of in vitro efficacy is certainly not all the time translated to in vivo. The NIH3T6.7 tumors grew so rapidly that AlBD/HER2Afb/RTA-KDEL was not potent sufficient to maintain the tumors in verify.
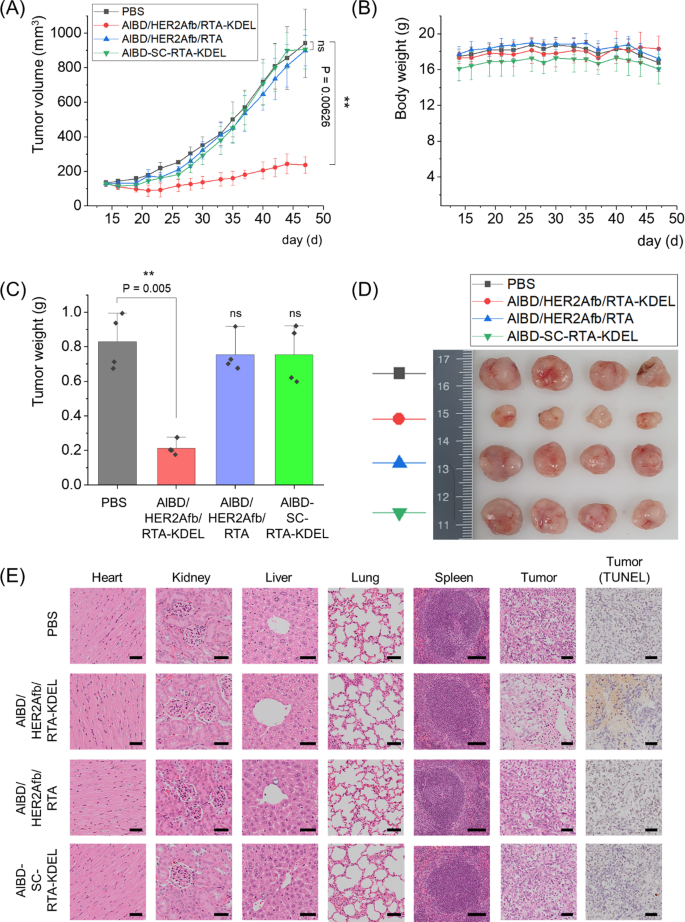
We subsequent xenografted human breast most cancers cells, SK-BR-3 cells, to immunodeficient nude mice and 5 μg of AlBD/HER2Afb/RTA-KDEL, AlBD/HER2Afb/RTA, AlBD-SC-RTA-KDEL or PBS have been intravenously injected when the typical tumor quantity reached 130 mm3, roughly. Every group was injected each two or three days for eight occasions, and physique weights and tumor volumes have been measured earlier than injection (Fig. 6A and B). Mice have been sacrificed at day 47, and main organs (coronary heart, lung, liver, kidney, and spleen) and tumor plenty have been biopsied (Fig. 6C–E). Much like in vitro knowledge, SK-BR-3 tumor development was dramatically suppressed solely upon the administration of AlBD/HER2Afb/RTA-KDEL, which has each the KDEL sign peptide and the focusing on ligand; both AlBD/HER2Afb/RTA (with out KDEL) or AlBD-SC-RTA-KDEL (with out focusing on ligand) didn’t affect SK-BR-3 tumor development in any respect (Fig. 6A–D). There was no noticeable physique weight change noticed (Fig. 6B). Hematoxylin and eosin (H&E)-stained tissue sections of biopsied organs didn’t present any noticeable distinction in morphology and construction of main organs among the many totally different mice therapy teams, suggesting that there was no vital side-effect or off-target harm trigger by RTA variants in main organs. Nonetheless, H&E-stained tumor sections of the mice handled with AlBD/HER2Afb/RTA-KDEL confirmed a a lot loosely packed construction in contrast with the opposite tumor sections (Fig. 6E). Moreover, terminal deoxynucleotidyl transferase dUTP nick finish labeling (TUNEL) assay of tumor sections of the mice handled with AlBD/HER2Afb/RTA-KDEL additionally confirmed excessive ranges of fragmented DNA (Fig. 6E, brown stain), whereas no noticeable DNA fragmentation was noticed within the tumor sections of mice from different therapy teams (Fig. 6E). Taken collectively, these outcomes implicate that AlBD/HER2Afb/RTA-KDEL circulated within the blood stream of the mice for a very long time, particularly delivered to the SK-BR-3 tumor web site, efficiently entered the cells, and successfully suppressed SK-BR-3 tumor development by inducing apoptotic cell demise. Efficient tumor suppression was attainable solely when the focusing on ligand and the KDEL sign peptide concurrently existed with the RTA.
In vivo therapeutic efficacy of AlBD/HER2Afb/RTA-KDEL and different AlBD-containing RITs in SK-BR-3 tumor-bearing mice. A Tumor volumes and B physique weights have been measured with a caliper and scaler, respectively. C Weight of biopsied tumors have been measured and D an image of the biopsied tumors are proven. E The hearts, kidneys, livers, lungs, spleens, and tumors collected from sacrificed mice handled with PBS, AlBD/HER2Afb/RTA-KDEL, AlBD/HER2Afb/RTA, or AlBD-SC-RTA-KDEL have been fastened, embedded in paraffin, and sectioned. The organ tissue and tumor sections have been stained with hematoxylin and eosin (H&E) and further tumor sections have been independently stained with TUNEL. Photos have been captured utilizing an Olympus digital microscope. Scale bar, 100 μm at spleen and 50 μm at others. All knowledge proven in A–C are the means ± SD; n = 4 per group; **p < 0.01; ns not vital
There have been quite a few experiences on ricin-based RITs [38,39,40]. Though they’ve been actively developed towards Hodgkin and non-Hodgkin lymphoma together with scientific research [41,42,43,44] in addition to stable tumors preclinically [45,46,47], their anti-cancer efficacy was quite average. When the RTB was changed with one other focusing on moiety, ricin-based RITs have been much less environment friendly as a result of the RTB has roles in intracellular routing to the ER in addition to binding to cell floor galactoses [3, 40]. Alternatively, the RITs with the holotoxin have been typically much less particular than these with the RTA alone, leading to off-target results [3]. Based mostly on our outcomes, we suggest a working mannequin for intracellular supply of ricin-based RITs constructed on this research. When each the focusing on ligand and the KDEL sign peptide are fused to the RTA, it undergoes receptor-mediated endocytosis upon cancer-cell binding, follows a retrograde transport pathway guided by the KDEL sign peptide to succeed in the ER [8], and is subsequently launched to the cytosol by the ERAD pathway [3, 9], leading to apoptotic cell demise (Fig. 7, left half). In distinction, the RTA with the focusing on ligand alone can nonetheless be endocytosed, however most of them might be recycled again to the cell floor to be faraway from the cells, leading to decreased therapeutic efficacy (Fig. 7, proper half).
Though it has been identified that the KDEL sign peptide directs protein toxins to the ER and improves the cytotoxicity of the RTA [23, 48, 49], most earlier research demonstrated their cytotoxic efficacy in vitro quite than in vivo. Right here, we constructed ricin-based RITs utilizing each affibody molecule-based focusing on ligands and the KDEL sign peptide. We efficiently confirmed their glorious target-specific cytotoxic efficacy in vivo with the help of albumin-binding area. These findings provide a possible technique to extend therapeutic efficacy of protein-based immunotoxins by rationally designing intracellular routes and incorporating guiding sequences to beat the drug tolerance of tumors.